Preclinical Safety (Coursera)
Categories
Effort
Languages
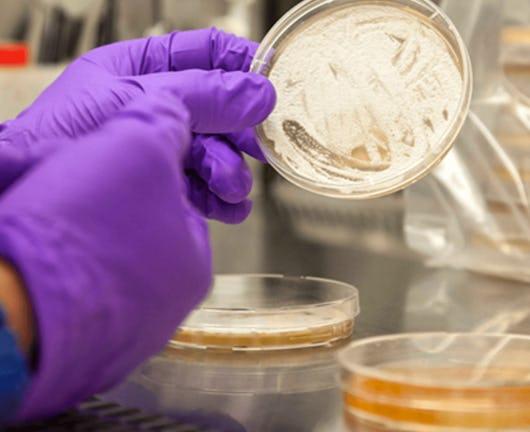
Patient safety is of paramount importance for any drug discovery program. This course looks at some of the lessons learned which have influenced how promising molecules are currently evaluated for safety risks. In vitro and in vivo toxicology and safety studies are discussed, why they are performed, and how [...]
May 20th 2024